Yet few motorists know how they work or how to maintain them.
A battery has two terminals, one marked positive (+) and one negative (-) to which are connected to leads that run to the ignition, starter motor, lights and other electrical components.
Inside the typical lead acid battery are lead plates in electrolyte liquid which creates an electro-chemical reaction to produce a charge to the battery terminals.
These batteries can get a build-up of lead oxide on the terminals, which need to be cleaned for optimum performance. You should also keep the electrolyte topped up with de-mineralised water, not tap water or acid. Don’t overfill.
Batteries are recharged by the alternator, which turns mechanical energy into an electrical charge. A failed alternator can allow the battery to go flat. In this case the battery can be recharged however fully depleted batteries will have a shorter life.
 Most modern car batteries are either absorbed glass mat or gel cell. These are sealed for life requiring no maintenance. When they go flat, you simply replace them.
Most modern car batteries are either absorbed glass mat or gel cell. These are sealed for life requiring no maintenance. When they go flat, you simply replace them.
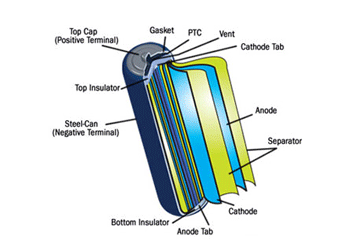
Electric cars and some luxury models use lightweight lithium-ion batteries (See image to the right), which use lithium-ion instead of electrolyte liquid to create the charge.





.jpg)
.jpg)

.jpg)


.jpg)

.jpg)





.jpg)



.jpg)



